Films and fussy eaters at the University of Duisburg-Essen Biofilm Centre. Impressions from a tour of their laboratories with Isabelle Heker and interesting conversations along the way. The department specialises in working on aquatic micro-organisms, which can range from pathogens to bugs that can clean up the environment. One of the features of many aquatic micro-organisms is that they live in Biofilms, strange and complex environments that can dramatically alter the properties of the organisms within them.
The initial intention was to visit my friends, the Hekers, for a bit of photography for an afternoon. Daughter Isabelle Heker invited me to come along and see the work that she and others were conducting at the labs in the University of Duisburg-Essen Biofilm Centre.
The obligatory safety talk with Dr Jost Wingender, Head of the Department of Pathogens in Biofilms, brought back memories of when I was a Biological Safety Officer for a biotech company! Inevitably, it segued into conversations about Dr Wingender’s work. One of his interests is viruses that attack bacteria, also known as phages, and their behaviour in biofilms.
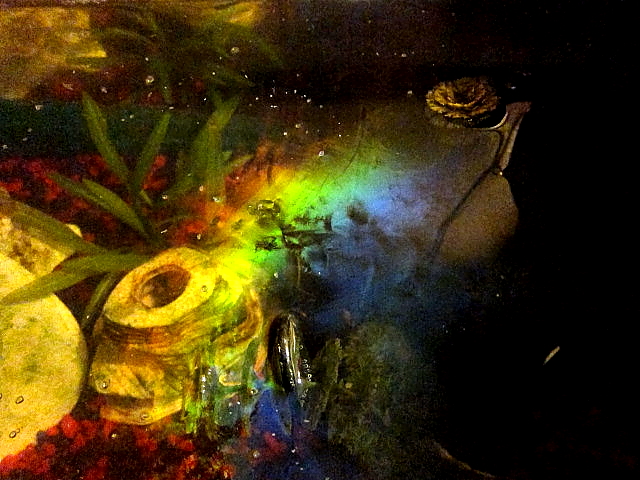 |
| An iridescent biofilm on the surface of a fish tank (Wikipedia) |
Many micro-organisms, including bacteria, live in biofilms, making rocks slippery, furring the leaves of water plants or coating your teeth with plaque.
They are often surrounded by a slime, whose composition can vary according to the species and the substrate they are on. The scientific term for this slime is EPS, short for extracellular polymeric substances.
Just like there is a whole ecology and range of environments in a forest, that changes from the soil to the tops of the tree canopy, there is considerable variation in the EPS from its external interface with water to the bottom layer, adhering to a substrate. There are gradients of oxygen, nutrients and acidity.
In the wild, the ecosystems within the EPS are created by different microscopic species either helping each other by producing complementary nutrients or degrading toxins and natural antibiotics – or they can be in competition for food and resources.
Back to phages and viruses. The EPS also binds contaminants and viruses, concentrating them. As this can happen with human pathogens, it means that biofilms could be far more infectious than the more diluted pathogens free-floating in water. Suddenly, Dr Wingender’s work is a lot more relevant to us.
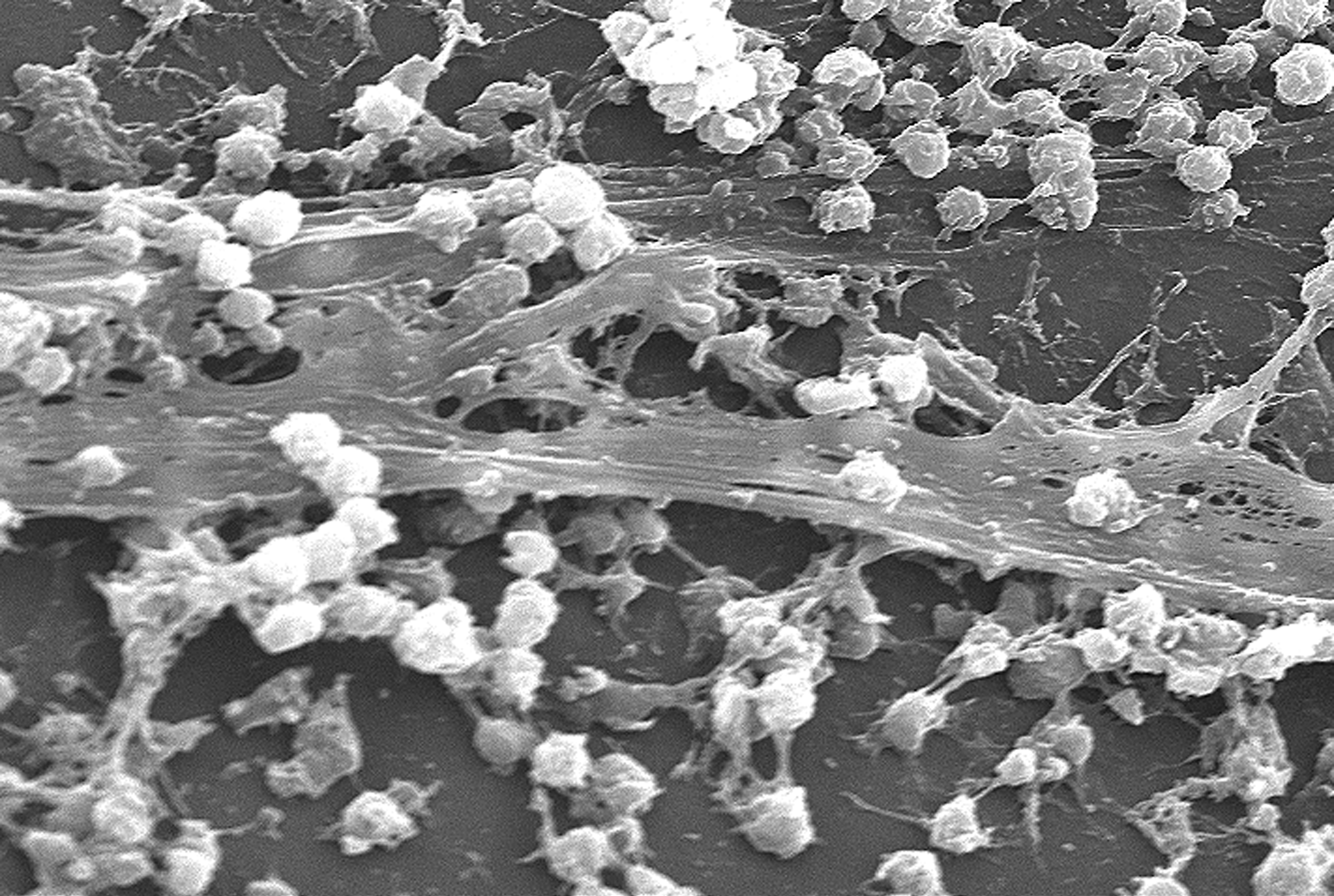 |
| S.aureus biofilm on an indwelling catheter (Wikipedia) |
On to the lab tour. Dr Martin Mackowiak led and also talked a bit about his work. Knowing what species of micro-organisms are present in a biofilm and how many there are is not that easy. Furthermore, the populations can change with age. Imagine being in a rocket up in space looking down on to the world below. With a high powered telescope you might just about make out moving shapes as tiny dots, it is hard to distinguish between say the people, pigeons and pets out in a city or between different farm animals out in the fields.
Fortunately, micro-organisms contain DNA. What’s more, the EPS, the slime also contains fragments of DNA, from dying cells or even deliberately secreted. The DNA will be present in very small quantities. However, using a method familiar from my lab days, quantitative PCR, you can get a good measure of how many of the scant DNA molecules from different species are present. Martin had just harvested a batch of biofilm he’d cultured over weeks in readiness for analysis. It was also fun to see equipment gathering dust that in my day we would have drooled over, like the Pulsed Field Gel Electrophoresis kit, and machines that were still very familiar to me in current use.
Most importantly, Isabelle was able to tell me about her graduate project. Of the myriads of species of bacteria in water, there are many fussy eaters, of which some are able to degrade pollutants, such as hydrocarbons. It’s vital to understand how they do this before scaling up any method. Isabelle’s project was to identify some of the breakdown products from one pollutant.
First, she had to purify and separate the different breakdown products by size and chemical properties using liquid chromatography – the basic principle is similar to separating the colours out of different felt-tip pens on tissue paper.
Image on left is an example of different leaf pigments separated by chromatography on a plate, CC Flo~commonswiki. Isabelle was using special columns in her work.
The mass of each separated breakdown product was then determined using a
mass spectrometer. Each molecule is vaporised, ionised to give it an electric charge and then accelerated toward a detector through a magnetic field. The charged molecules get separated by charge and weight during their flight through the magnetic field and then hit the detector. You can very accurately determine how heavy a molecule is. Isabelle was now at the interesting stage of knowing what sizes of products she is getting.
The puzzle to be solved now is: WHICH is the most likely chemical formula of a breakdown product where there are several with the same mass? This requires a good knowledge of chemistry and logical problem solving. Fortunately, Isabelle is enjoying the challenge and hopes to go on to do a masters in the same department.
This was a thoroughly enjoyable couple of hours catching up with the new field of water and biofilm biologies. Thank you to Isabelle and all at the University of Duisburg-Essen Biofilm Centre for sharing their time and interests.
Now it was time to go off to meet the others for a glorious walk along the beautiful transformed River Ruhr - full of aquatic life and, of course, Biofilms.









